Lesson 2: Regulations Versus Statutes
Topic 1: Statutes Authorize Regulations
U.S. importation regulations are not laws enacted by the U.S. Congress, but they do have the force of law. In this topic, you will learn how and why this is the case. You will also learn about the two principal statutes under which APHIS’ regulations are authorized.
Objectives:
- Describe how and why Congress delegates authority to the U.S. Department of Agriculture and to APHIS
- Describe how and why regulations are developed to prevent the introduction of animal and plant pests and diseases
- Describe APHIS’ main authorizing statutes and the principal goals they set
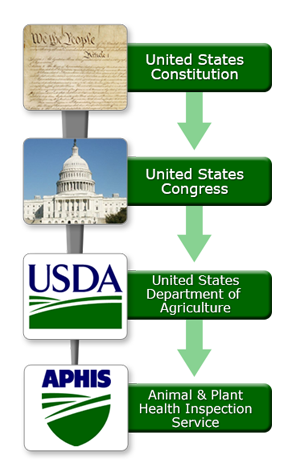
In the United States, the U.S. Congress has the power to enact statutes to impose restrictions on the importation of plants, animals, their products, and any other organisms or articles that could pose a risk to plants and animals in the United States. Congress is appointed this power through the U.S. Constitution. First, Congress writes and passes a statute. The statute is then signed into law by the President of the United States. This provides authority for federal agencies, such as the United States Department of Agriculture (USDA), to write regulations to apply the statute. An agency is an authority within the executive branch of the U.S. Government that holds jurisdiction over a specific issue area. For example, the U.S. Department of Agriculture implements the laws Congress passes that are related to agriculture.
Congress has the authority to set detailed regulatory standards, but it often elects not to use that authority. Instead, Congress sets broad goals and then instructs agencies in the executive branch of the U.S. government to determine the best way to meet them. Congress has chosen this strategy of delegation with respect to animal and plant health issues.
Congress has delegated authority on animal and plant health issues to the Secretary of the USDA. The Secretary of the USDA has, in turn, delegated authority on animal and plant health issues to the Animal and Plant Health Inspection Service (APHIS). Thus, Congress establishes the framework and goals for U.S. policy on animal and plant health issues. Within APHIS, the Plant Protection and Quarantine program (PPQ) acts as the national plant protection organization (NPPO) under the IPPC, and the Veterinary Services program (VS) acts as the competent veterinary authority under the OIE.
In order to achieve Congress’ goals, APHIS promulgates regulations. Regulations are requirements that are created by an Agency, and that carry the force of law. They are also referred to as “rules”. Promulgating a regulation, or rule, means to put it into effect by official proclamation. We will discuss how “official proclamation” works in the U.S. regulatory process later in this module.
The U.S. Congress passes statutes that authorize APHIS, through the Secretary of the USDA, to take actions to achieve the goals set out in the animal and plant health statutes. Regulations promulgated by APHIS to meet these goals have the force of law. For example, APHIS might create a regulation that requires guavas imported from an area where fruit flies are present to be treated for fruit flies, and requires the importer to document that the treatment has been performed. This regulation has the force of law. People who then try to import guavas into the United States without first having them treated in accordance with APHIS regulations may face civil and criminal penalties in U.S. courts.
APHIS has two major statutory authorities that govern imports of animals and plants and their products. The Plant Protection Act (PPA) provides APHIS with the authority to impose restrictions on the importation of fruits and vegetables, as well as other articles whose importation could affect plant health. The Animal Health Protection Act (AHPA) focuses on animal health risks, including the importation of animals and animal products. We will discuss those more in depth now.
Taken together, these statutes give APHIS the authority it needs to take action consistent with its commitments as a signatory to the SPS Agreement.
It is important to note that an agency can only issue regulations within the scope of its authorizing statutes. For example, APHIS cannot restrict imports based on their potential human health implications. Human health issues are handled by the Food and Drug Administration (FDA) and the USDA’s Food Safety and Inspection Service (FSIS). The importation of meat into the United States is routinely subject to both APHIS and FSIS supervision. The Environmental Protection Agency (EPA) handles issues of allowable pesticide residues for imported fruits and vegetables.
Countries seeking market access for animals, plants, and their products need to work with every relevant agency of the U.S. government to satisfy the applicable requirements.
In addition, APHIS regulates the importation of genetically modified organisms that could be plant or animal pests under the PPA and AHPA through its Biotechnology Regulatory Services (BRS) program. Market access requests for such organisms would not only be subject to APHIS’ general plant or animal health requirements under those statutes but also to BRS regulations.
Congress delegates authority to agencies to promulgate regulations. Most market access requests related to SPS issues are governed by regulations promulgated under the Plant Protection Act or the Animal Health Protection Act. These statutes explicitly address market access among their provisions and indicate that facilitating trade is one goal of SPS regulation.
To continue, select Topic 2 from the Topics menu above or click here.